Author Information
Ashish Zarariya*, Rajshree Dayanand.Katke**,Preeti Lewis***, Grishma.D.Agrawal****
(*Associate
Professor, ** Medical Superintendant & Associate Professor,
*** Assistant Professor, **** Third Year Resident. Department of Obstetrics
and Gynaecology, Cama And Albless Hospitals, Grant Government
Medical College, Mumbai, India.)
Abstract
We
report an interesting case of advanced rhabdomyosarcoma
(RMS) in a
teenage pregnancy leading to mortality. A 19 year old married girl presented
with 8 months of amenorrhea and a wart like perianal lesion.
She was
lost follow up for a month and came in emergency in critical condition with septicaemia, hyperkalemia
and acute renal failure (ARF). The
wart sized
lesion had progressed in a month to gross perianal mass which was extending
inside pelvis up to the lower lumbar region. The patient succumbed within 8 hours
of admission. On
post-mortem histopathological examination, the lesion was diagnosed as a rhabdomyosarcoma.
Introduction
A case of rhabdomyosarcoma with near term pregnancy is a
exceedingly rare event. Cancer in pregnancy itself is relatively rare. Most
frequently reported sites are breast, head and neck, lymphomas and melenomas.[1] The origin of RMS is from tissue that imitates normal striated muscle.[2] Common sites are head and neck, genitourinary tract,
thorax and abdomen. RMS is classified by international classification as
embryonal, botryoid, spindle cell, alveolar, and undifferentiated. Out of this
the botryoid and spindle cell types,
considered to be the subtypes of embroyonal
RMS, occur in 0-10 age group and have superior
prognosis.[3] Alveolar
RMS has poor prognosis, and its incidence is evenly distributed in 0-19 age
group.[4]
RMS is classified by international classification as Embryonal and
Alveolar.
Out of this Botryoid and Spindle cell, considered to be the subtypes of
Embroyonal.[3]
Case report
A 19 year
old Primigravida Unregistered, came to ANC OPD for Registration with 7 months amenorrhea and perianal wart
like growth.
Figure 1. Perianal lesion at the first visit.
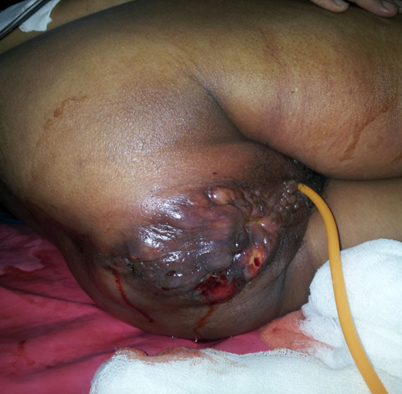
Later
after 1 month, the patient presented in a critical state in emergency, with palpable 3-4 cm hard lump in
the left
breast, edema in both lower limb up to thighs and indurated ulcerative growth with
multiple grouped vesicles present around anus. The growth was extending
inside the pelvis
with gross left inguinal lymphadenopathy.
Figure 2. Perianal lesion at the second visit.
She had an intrauterine fetal death at 34-36 weeks. On per vaginal examination, the cervix could not felt due to the growth. She was treated with supportive line of management. Her investigations were suggestive of hyperkalemia, septicemia and ARF. She died within 8 hours of admission. Her postmortem examination showed a palpable lymph node in left inguinal region 3 cm in diameter, swelling of the left breast with palpable lymph node 2 cm diameter, a 10x8 cm whitish colored growth in the left lower lumbar region and left side of the pelvis. It extended to the perianal region in the form of nodules. The uterus showed features of acute myometritis with degenerated decidua.
She had an intrauterine fetal death at 34-36 weeks. On per vaginal examination, the cervix could not felt due to the growth. She was treated with supportive line of management. Her investigations were suggestive of hyperkalemia, septicemia and ARF. She died within 8 hours of admission. Her postmortem examination showed a palpable lymph node in left inguinal region 3 cm in diameter, swelling of the left breast with palpable lymph node 2 cm diameter, a 10x8 cm whitish colored growth in the left lower lumbar region and left side of the pelvis. It extended to the perianal region in the form of nodules. The uterus showed features of acute myometritis with degenerated decidua.
Figure 3: Postmortem abdominal gross findings.
Histopathological examination
of the tumor mass around
the vertebral column showed a high grade malignant tumor with
small round
tumor cells with rhabdoid differentiation- a rhadomyosarcoma.
A part of uterus showed infiltration and abscess
formation, acute myometritis and degenerated decidua.
Discussion
The
incidence of RMS is very low. Due to its rarity and diagnostic
diversity, very little is known about the etiology of RMS. Several environmental factors have increased risk
of developing RMS, such
as paternal cigarette smoking,[5] advanced maternal age and x-ray exposure in utero,[6] maternal and child’s antibiotic use,[7]
stillbirths[8] and
maternal recreational drug use[9]. In
addition genetic changes may also play an important role in RMS development. Familial
syndromes associated with inherited gene defects, like Li-Fraumeni syndrome and
neurofibromatosis, have been associated with RMS.[10] RMS
relative 5-year survival rates have not increased significantly over the past
30 years; RMS
has one of the worst prognosis with high rates of mortalities. The
diagnosis
of a rhabdomyosarcoma depends on recognition of differentiation
of its cells
toward skeletal muscle cells. Immunohistochemical marker of rhabdomyosarcoma
are MyoD1 and Myogenin. In our case immunohistochemistry could not be done as
it was a post-mortem case and the facility was not available in our institute.
Pertaining
to our case a diagnosis of malignancy must be kept in mind for a painful
ulcerative growth in this age group.Our patient presented as a teenage
pregnancy and so the tumor was all the more rapidly progressive in nature
leading to catastrophic, life threatening events ultimately resulting in
untimely mortality of the patient.
References
1.
Donegan Wl: Cancer and pregnancy.
CA Cancer J Clin 1983;33:194-214.
2.
Stout AP. Rhabdomyosarcoma of the Skeletal
Muscles. Ann Surg. 1946;123:447–472.
3.
Gurney JG, Young JL, Roffers SD, Smith MA, Bunin
GR. SEER Pediatric Monograph. National Cancer Institute; 2005. Soft
Tissue Sarcomas.
4.
Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T,
Young JL, Bunin GR, editors. Cancer incidence and survival among children
and adolescents: United States SEER Program 1975–1995. Bethesda: National
Cancer Institute;1999.
5.
Gurney JG, Severson RK, Davis S, et al.:
Incidence of cancer in children in the United States. Sex-, race-, and 1-year
age-specific rates by histologic type. Cancer 1995;75(8): 2186-95.
6.
Ries LA, Kosary CL, Hankey BF, et al., eds.:
SEER Cancer Statistics Review, 1973-1996. Bethesda, Md: National Cancer
Institute, 1999.
7.
Grufferman S, Wang
HH, DeLong ER, Kimm SY, Delzell ES, Falletta JM. Environmental factors in the
etiology of rhabdomyosarcoma in childhood. J Natl Cancer Inst 1982;68:107–113.
8.
Grufferman S, Gula
MJ, Olshan AF, Falletta JM, Pendergrass TW, Buckley J, Maurer HM. In utero
x-ray exposure and risk of childhood rhabdomyosarcoma. Paediatr Perinat
Epidemiol 1991;5:A6.
9.
Hartley AL, Birch JM,
McKinney PA, Teare MD, Blair V, Carrette J, Mann JR, Draper GJ, Stiller CA,
Johnston HE, et al. The Inter-Regional Epidemiological Study of Childhood
Cancer (IRESCC): case control study of children with bone and soft tissue
sarcomas. Br J Cancer 1988;58:838–842.
10. 10. Ghali MH, Yoo KY, Flannery JT, Dubrow R. Association between
childhood rhabdomyosarcoma and maternal history of stillbirths. Int J
Cancer 1992;50:365–368.
Citation
Zarariya A, Katke RD